Protein–protein interaction

The horseshoe shaped ribonuclease inhibitor (shown as wireframe) forms a protein–protein interaction with the ribonuclease protein. The contacts between the two proteins are shown as coloured patches.
Protein–protein interactions (PPIs) are the physical contacts of high specificity established between two or more protein molecules as a result of biochemical events steered by electrostatic forces including the hydrophobic effect. Many are physical contacts with molecular associations between chains that occur in a cell or in a living organism in a specific biomolecular context.[1]
Proteins rarely act alone as their functions tend to be regulated. Many molecular processes within a cell are carried out by molecular machines that are built from a large number of protein components organized by their PPIs. These interactions make up the so-called interactomics of the organism, while aberrant PPIs are the basis of multiple aggregation-related diseases, such as Creutzfeldt–Jakob, Alzheimer's diseases, and may lead to cancer.
PPIs have been studied from different perspectives: biochemistry, quantum chemistry, molecular dynamics, signal transduction, among others.[2] All this information enables the creation of large protein interaction networks – similar to metabolic or genetic/epigenetic networks – that empower the current knowledge on biochemical cascades and molecular etiology of disease, as well as the discovery of putative protein targets of therapeutic interest.
Contents
1 Examples
1.1 Electron transfer proteins
1.2 Signal transduction
1.3 Transport across membranes
1.4 Cell metabolism
1.5 Muscle contraction
2 Types
2.1 Homo-oligomers vs. hetero-oligomers
2.2 Stable interactions vs. transient interactions
2.3 Covalent vs. non-covalent
2.4 Role of water
3 Structure
3.1 Domains
4 Properties of the interface
5 Regulation
6 Methods
6.1 Yeast two-hybrid screening
6.2 Affinity purification coupled to mass spectrometry
6.3 Other potential methods
6.4 Text mining methods
6.5 Machine learning methods
7 Databases
8 Interaction networks
8.1 Signed interaction networks
8.1.1 RNA interference screens
9 As therapeutic targets
10 See also
11 References
12 Further reading
13 External links
Examples
Electron transfer proteins
In many metabolic reactions, a protein that acts as an electron carrier binds to an enzyme that acts its reductase. After it receives an electron, it dissociates and then binds to the next enzyme that acts its oxidase (i.e. acceptor of the electron). These interactions between proteins are dependent on highly specific binding between proteins to ensure efficient electron transfer. Examples: mitochondrial oxidative phosphorylation chain system components cytochrome c-reductase / cytochrome c / cytochrome c oxidase; microsomal and mitochondrial P450 systems.[3]
In the case of the mitochondrial P450 systems, the specific residues involved in the binding of the electron transfer protein adrenodoxin to its reductase were identified as two basic Arg residues on the surface of the reductase and two acidic Asp residues on the adrenodoxin.[4]
More recent work on the phylogeny of the reductase has shown that these residues involved in protein-protein interactions have been conserved throughout the evolution of this enzyme.[5]
Signal transduction
The activity of the cell is regulated by extracellular signals. Signal propagation inside and/or along the interior of cells depends on PPIs between the various signaling molecules. The recruitment of signaling pathways through PPIs is called signal transduction and plays a fundamental role in many biological processes and in many diseases including Parkinson's disease and cancer.
Transport across membranes
A protein may be carrying another protein (for example, from cytoplasm to nucleus or vice versa in the case of the nuclear pore importins).
Cell metabolism
In many biosynthetic processes enzymes interact with each other to produce small compounds or other macromolecules.
Muscle contraction
Physiology of muscle contraction involves several interactions. Myosin filaments act as molecular motors and by binding to actin enables filament sliding.[6] Furthermore, members of the skeletal muscle lipid droplet-associated proteins family associate with other proteins, as activator of adipose triglyceride lipase and its coactivator comparative gene identification-58, to regulate lipolysis in skeletal muscle.[7]
Types
To describe the types of protein–protein interactions (PPIs) it is important to consider that proteins can interact in a "transient" way (to produce some specific effect in a short time) or to interact with other proteins in a "stable" way to build multiprotein complexes that are molecular machines within the living systems. A protein complex assembly can result in the formation of homo-oligomeric or hetero-oligomeric complexes. In addition to the conventional complexes, as enzyme-inhibitor and antibody-antigen, interactions can also be established between domain-domain and domain-peptide. Another important distinction to identify protein-protein interactions is the way they have been determined, since there are techniques that measure direct physical interactions between protein pairs, named “binary” methods, while there are other techniques that measure physical interactions among groups of proteins, without pairwise determination of protein partners, named “co-complex” methods.[1]
Homo-oligomers vs. hetero-oligomers
Homo-oligomers are macromolecular complexes constituted by only one type of protein subunit. Protein subunits assembly is guided by the establishment of non-covalent interactions in the quaternary structure of the protein. Disruption of homo-oligomers in order to return to the initial individual monomers often requires denaturation of the complex.[8] Several enzymes, carrier proteins, scaffolding proteins, and transcriptional regulatory factors carry out their functions as homo-oligomers.
Distinct protein subunits interact in hetero-oligomers, which are essential to control several cellular functions. The importance of the communication between heterologous proteins is even more evident during cell signaling events and such interactions are only possible due to structural domains within the proteins (as described below).
Stable interactions vs. transient interactions
Stable interactions involve proteins that interact for a long time, taking part of permanent complexes as subunits, in order to carry out structural or functional roles. These are usually the case of homo-oligomers (e.g. cytochrome c), and some hetero-oligomeric proteins, as the subunits of ATPase. On the other hand, a protein may interact briefly and in a reversible manner with other proteins in only certain cellular contexts – cell type, cell cycle stage, external factors, presence of other binding proteins, etc. – as it happens with most of the proteins involved in biochemical cascades. These are called transient interactions. For example, some G protein-coupled receptors only transiently bind to Gi/o proteins when they are activated by extracellular ligands,[9] while some Gq-coupled receptors, such as muscarinic receptor M3, pre-couple with Gq proteins prior to the receptor-ligand binding.[10] Interactions between intrinsically disordered protein regions to globular protein domains (i.e. MoRFs) are transient interactions.[11]
Covalent vs. non-covalent
Covalent interactions are those with the strongest association and are formed by disulphide bonds or electron sharing. Although being rare, these interactions are determinant in some posttranslational modifications, as ubiquitination and SUMOylation. Non-covalent bonds are usually established during transient interactions by the combination of weaker bonds, such as hydrogen bonds, ionic interactions, Van der Waals forces, or hydrophobic bonds.[12]
Role of water
Water molecules play a significant role in the interactions between proteins.[13][14] The crystal structures of complexes, obtained at high resolution from different but homologous proteins, have shown that some interface water molecules are conserved between homologous complexes. The majority of the interface water molecules make hydrogen bonds with both partners of each complex. Some interface amino acid residues or atomic groups of one protein partner engage in both direct and water mediated interactions with the other protein partner. Doubly indirect interactions, mediated by two water molecules, are more numerous in the homologous complexes of low affinity.[15] Carefully conducted mutagenesis experiments, e.g. changing a tyrosine residue into a phenylalanine, have shown that water mediated interactions can contribute to the energy of interaction.[16] Thus, water molecules may facilitate the interactions and cross-recognitions between proteins.
Structure

Crystal structure of modified Gramicidin S horizontally determined by X-ray crystallography

NMR structure of cytochrome C illustrating its dynamics in solution
The molecular structures of many protein complexes have been unlocked by the technique of X-ray crystallography.[17][18] The first structure to be solved by this method was that of sperm whale myoglobin by Sir John Cowdery Kendrew.[19] In this technique the angles and intensities of a beam of X-rays diffracted by crystalline atoms are detected in a film, thus producing a three-dimensional picture of the density of electrons within the crystal.[20]
Later, nuclear magnetic resonance also started to be applied with the aim of unravelling the molecular structure of protein complexes. One of the first examples was the structure of calmodulin-binding domains bound to calmodulin.[18][21] This technique is based on the study of magnetic properties of atomic nuclei, thus determining physical and chemical properties of the correspondent atoms or the molecules. Nuclear magnetic resonance is advantageous for characterizing weak PPIs.[22]
Domains
Proteins hold structural domains that allow their interaction with and bind to specific sequences on other proteins:
Src homology 2 (SH2) domain
- SH2 domains are structurally composed by three-stranded twisted beta sheet sandwiched flanked by two alpha-helices. The existence of a deep binding pocket with high affinity for phosphotyrosine, but not for phosphoserine or phosphothreonine, is essential for the recognition of tyrosine phosphorylated proteins, mainly autophosphorylated growth factor receptors. Growth factor receptor binding proteins and phospholipase Cγ are examples of proteins that have SH2 domains.[23]
Src homology 3 (SH3) domain
- Structurally, SH3 domains are constituted by a beta barrel formed by two orthogonal beta sheets and three anti-parallel beta strands. These domains recognize proline enriched sequences, as polyproline type II helical structure (PXXP motifs)[verification needed] in cell signaling proteins like protein tyrosine kinases and the growth factor receptor bound protein 2 (Grb2).[23]
Phosphotyrosine-binding (PTB) domain
- PTB domains interact with sequences that contain a phosphotyrosine group. These domains can be found in the insulin receptor substrate.[23]
LIM domain
- LIM domains were initially identified in three homeodomain transcription factors (lin11, is11, and mec3). In addition to this homeodomain proteins and other proteins involved in development, LIM domains have also been identified in non-homeodomain proteins with relevant roles in cellular differentiation, association with cytoskeleton and senescence. These domains contain a tandem cysteine-rich Zn2+-finger motif and embrace the consensus sequence CX2CX16-23HX2CX2CX2CX16-21CX2C/H/D. LIM domains bind to PDZ domains, bHLH transcription factors, and other LIM domains.[23]
Sterile alpha motif (SAM) domain
- SAM domains are composed by five helices forming a compact package with a conserved hydrophobic core. These domains, which can be found in the Eph receptor and the stromal interaction molecule (STIM) for example, bind to non-SAM domain-containing proteins and they also appear to have the ability to bind RNA.[23]
PDZ domain
- PDZ domains were first identified in three guanylate kinases: PSD-95, DlgA and ZO-1. These domains recognize carboxy-terminal tri-peptide motifs (S/TXV), other PDZ domains or LIM domains and bind them through a short peptide sequence that has a C-terminal hydrophobic residue. Some of the proteins identified as having PDZ domains are scaffolding proteins or seem to be involved in ion receptor assembling and receptor-enzyme complexes formation.[23]
FERM domain
- FERM domains contain basic residues capable of binding PtdIns(4,5)P2. Talin and focal adhesion kinase (FAK) are two of the proteins that present FERM domains.[23]
Calponin homology (CH) domain
- CH domains are mainly present in cytoskeletal proteins as parvin.[23]
Pleckstrin homology domain
- Pleckstrin homology domains bind to phosphoinositides and acid domains in signaling proteins.
WW domain
- WW domains bind to proline enriched sequences.
- WSxWS motif
- Found in cytokine receptors
Properties of the interface
The study of the molecular structure can give fine details about the interface that enables the interaction between proteins. When characterizing PPI interfaces it is important to take into account the type of complex.[8]
Parameters evaluated include size (measured in absolute dimensions Å2 or in solvent-accessible surface area (SASA)), shape, complementarity between surfaces, residue interface propensities, hydrophobicity, segmentation and secondary structure, and conformational changes on complex formation.[8]
The great majority of PPI interfaces reflects the composition of protein surfaces, rather than the protein cores, in spite of being frequently enriched in hydrophobic residues, particularly in aromatic residues.[24] PPI interfaces are dynamic and frequently planar, although they can be globular and protruding as well.[25] Based on three structures – insulin dimer, trypsin-pancreatic trypsin inhibitor complex, and oxyhaemoglobin – Cyrus Chothia and Joel Janin found that between 1,130 and 1,720 Å2 of surface area was removed from contact with water indicating that hydrophobicity is a major factor of stabilization of PPIs.[26] Later studies refined the buried surface area of the majority of interactions to 1,600±350 Å2. However, much larger interaction interfaces were also observed and were associated with significant changes in conformation of one of the interaction partners.[17] PPIs interfaces exhibit both shape and electrostatic complementarity.[8][27]
Regulation
- Protein concentration, which in turn are affected by expression levels and degradation rates;
- Protein affinity for proteins or other binding ligands;
- Ligands concentrations (substrates, ions, etc.);
- Presence of other proteins, nucleic acids, and ions;
Electric fields around proteins.- Occurrence of covalent modifications;
Methods
There are a multitude of methods to detect them.[28] Each of the approaches has its own strengths and weaknesses, especially with regard to the sensitivity and specificity of the method. The most conventional and widely used high-throughput methods are yeast two-hybrid screening and affinity purification coupled to mass spectrometry.[1]

Principles of yeast and mammalian two-hybrid systems
Yeast two-hybrid screening
This system was firstly described in 1989 by Fields and Song using Saccharomyces cerevisiae as biological model.[29] Yeast two hybrid allows the identification of pairwise PPIs (binary method) in vivo, in which the two proteins are tested for biophysically direct interaction. The Y2H is based on the functional reconstitution of the yeast transcription factor Gal4 and subsequent activation of a selective reporter such as His3. To test two proteins for interaction, two protein expression constructs are made: one protein (X) is fused to the Gal4 DNA-binding domain (DB) and a second protein (Y) is fused to the Gal4 activation domain (AD). In the assay, yeast cells are transformed with these constructs. Transcription of reporter genes does not occur unless bait (DB-X) and prey (AD-Y) interact with each other and form a functional Gal4 transcription factor. Thus, the interaction between proteins can be inferred by the presence of the products resultant of the reporter gene expression.[12][30] In cases in which the reporter gene expresses enzymes that allow the yeast to synthesize essential amino acids or nucleotides, yeast growth under selective media conditions indicates that the two proteins tested are interacting.
Despite its usefulness, the yeast two-hybrid system has limitations. It uses yeast as main host system, which can be a problem when studying proteins that contain mammalian-specific post-translational modifications. The number of PPIs identified is usually low because of a high false negative rate;[31] and, understates membrane proteins, for example.[32][33]
In initial studies that utilized Y2H, proper controls for false positives (e.g. when DB-X activates the reporter gene without the presence of AD-Y) were frequently not done, leading to a higher than normal false positive rate. An empirical framework must be implemented to control for these false positives.[34] Limitations in lower coverage of membrane proteins have been overcoming by the emergence of yeast two-hybrid variants, such as the membrane yeast two-hybrid (MYTH)[33] and the split-ubiquitin system,[30] which are not limited to interactions that occur in the nucleus; and, the bacterial two-hybrid system, performed in bacteria;[35]

Principle of Tandem Affinity Purification
Affinity purification coupled to mass spectrometry
Affinity purification coupled to mass spectrometry mostly detects stable interactions and thus better indicates functional in vivo PPIs.[36][30] This method starts by purification of the tagged protein, which is expressed in the cell usually at in vivo concentrations, and its interacting proteins (affinity purification). One of the most advantageous and widely used method to purify proteins with very low contaminating background is the tandem affinity purification, developed by Bertrand Seraphin and Matthias Mann and respective colleagues. PPIs can then be quantitatively and qualitatively analysed by mass spectrometry using different methods: chemical incorporation, biological or metabolic incorporation (SILAC), and label-free methods.[8]
Other potential methods
Diverse techniques to identify PPIs have been emerging along with technology progression. These include co-immunoprecipitation, protein microarrays, analytical ultracentrifugation, light scattering, fluorescence spectroscopy, luminescence-based mammalian interactome mapping (LUMIER), resonance-energy transfer systems, mammalian protein–protein interaction trap, electro-switchable biosurfaces, protein-fragment complementation assay, as well as real-time label-free measurements by surface plasmon resonance, and calorimetry.[32][33]
Text mining methods

Text mining protocol.
Publicly available information from biomedical documents is readily accessible through the internet and is becoming a powerful resource for collecting known protein-protein interactions (PPIs), PPI prediction and protein docking. Text mining is much less costly and time-consuming compared to other high-throughput techniques. Currently, text mining methods generally detect binary relations between interacting proteins from individual sentences using rule/pattern-based information extraction and machine learning approaches.[37] A wide variety of text mining applications for PPI extraction and/or prediction are available for public use, as well as repositories which often store manually validated and/or computationally predicted PPIs. Text mining can be implemented in two stages: information retrieval, where texts containing names of either or both interacting proteins are retrieved and information extraction, where targeted information (interacting proteins, implicated residues, interaction types, etc.) is extracted.
There are also studies using phylogenetic profiling, basing their functionalities on the theory that proteins involved in common pathways co-evolve in a correlated fashion across species. Some more complex text mining methodologies use advanced Natural Language Processing (NLP) techniques and build knowledge networks (for example, considering gene names as nodes and verbs as edges). Other developments involve kernel methods to predict protein interactions.[38]
Machine learning methods
These methods use machine learning to distinguish how interacting protein pairs differ from non-interacting protein pairs in terms of pairwise features such as cellular colocalization, gene co-expression, how closely located on a DNA are the genes that encode the two proteins, and so on.[39][40]Random Forest has been found to be most-effective machine learning method for protein interaction prediction.[41] Such methods have been applied for discovering protein interactions on human interactome, specifically the interactome of Membrane proteins[40] and the interactome of Schizophrenia-associated proteins.[39]
Databases
Large scale identification of PPIs generated hundreds of thousands interactions, which were collected together in specialized biological databases that are continuously updated in order to provide complete interactomes. The first of these databases was the Database of Interacting Proteins (DIP).[42] Since that time, the number of public databases has been increasing. Databases can be subdivided into primary databases, meta-databases, and prediction databases.[1]
Primary databases collect information about published PPIs proven to exist via small-scale or large-scale experimental methods. Examples: DIP, Biomolecular Interaction Network Database (BIND), Biological General Repository for Interaction Datasets (BioGRID), Human Protein Reference Database (HPRD), IntAct Molecular Interaction Database, Molecular Interactions Database (MINT), MIPS Protein Interaction Resource on Yeast (MIPS-MPact), and MIPS Mammalian Protein–Protein Interaction Database (MIPS-MPPI).[1]
Meta-databases normally result from the integration of primary databases information, but can also collect some original data. Examples: Agile Protein Interactomes Dataserver (APID),[43] The Microbial Protein Interaction Database (MPIDB),[44] Protein Interaction Network Analysis (PINA) platform, (GPS-Prot),[1] and Wiki-Pi.[45]
Prediction databases include many PPIs that are predicted using several techniques (main article). Examples: Human Protein–Protein Interaction Prediction Database (PIPs),[46] Interlogous Interaction Database (I2D), Known and Predicted Protein–Protein Interactions (STRING-db), and Unified Human Interactive (UniHI).[1]
Interaction networks
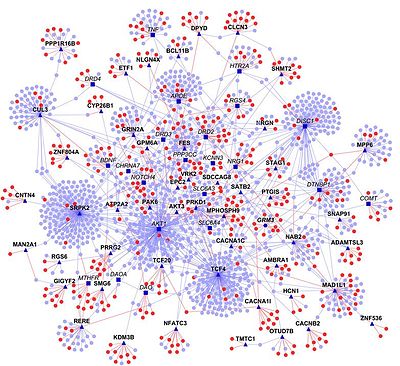
Schizophrenia PPI.[39]
Information found in PPIs databases supports the construction of interaction networks. Although the PPI network of a given query protein can be represented in textbooks, diagrams of whole cell PPIs are frankly complex and difficult to generate.
One example of a manually produced molecular interaction map is the Kurt Kohn's 1999 map of cell cycle control.[47] Drawing on Kohn's map, Schwikowski et al. in 2000 published a paper on PPIs in yeast, linking 1,548 interacting proteins determined by two-hybrid screening. They used a layered graph drawing method to find an initial placement of the nodes and then improved the layout using a force-based algorithm.[48][49]
Bioinformatic tools have been developed to simplify the difficult task of visualizing molecular interaction networks and complement them with other types of data. For instance, Cytoscape is an open-source software widely used and lots of plugins are currently available.[1][50] Pajek software is advantageous for the visualization and analysis of very large networks.[51]
Identification of functional modules in PPI networks is an important challenge in bioinformatics. Functional modules means a set of proteins that are highly connected to each other in PPI network. It is almost similar problem as community detection in social networks. There are some methods such as Jactive[52] modules and MoBaS.[53] Jactive modules integrate PPI network and gene expression data where as MoBaS integrate PPI network and Genome Wide association Studies.
The awareness of the major roles of PPIs in numerous physiological and pathological processes has been driving the challenge of unravel many interactomes. Examples of published interactomes are the thyroid specific DREAM interactome[54] and the PP1α interactome in human brain.[55]
Protein-protein relationships are often the result of multiple types of interactions or are deduced from different approaches, including co-localization, direct interaction, suppressive genetic interaction, additive genetic interaction, physical association, and other associations.[56]
Signed interaction networks

The protein protein interactions are displayed in a signed network that describes what type of interactions that are taking place[57]
Protein–protein interactions often result in one of the interacting proteins either being 'activated' or 'repressed'. Such effects can be indicated in a PPI network by "signs" (e.g. "activation" or "inhibition"). Although such attributes have been added to networks for a long time,[58] Vinayagam et al. (2014) coined the term Signed network for them. Signed networks are often expressed by labeling the interaction as either positive or negative. A positive interaction is one where the interaction results in one of the proteins being activated. Conversely a negative interaction indicates that one of the proteins being inactivated.[59]
Protein–protein interaction networks are often constructed as a result of lab experiments such as yeast two hybrid screens or 'affinity purification and subsequent mass spectrometry techniques.[60] However these methods do not provide the layer of information needed in order to determine what type of interaction is present in order to be able to attribute signs to the network diagrams.
RNA interference screens
RNA interference (RNAi) screens (repression of individual proteins between transcription and translation) are one method that can be utilized in the process of providing signs to the protein-protein interactions. Individual proteins are repressed and the resulting phenotypes are analyzed. A correlating phenotypic relationship (i.e. where the inhibition of either of two proteins results in the same phenotype) indicates a positive, or activating relationship. Phenotypes that do not correlate (i.e. where the inhibition of either of two proteins results in two different phenotypes) indicate a negative or inactivating relationship. If protein A is dependent on protein B for activation then the inhibition of either protein A or B will result in a cell losing the service that is provided by protein A and the phenotypes will be the same for the inhibition of either A or B. If, however, protein A is inactivated by protein B then the phenotypes will differ depending on which protein is inhibited (inhibit protein B and it can no longer inactivate protein A leaving A active however inactivate A and there is nothing for B to activate since A is inactive and the phenotype changes). Multiple RNAi screens need to be performed in order to reliably appoint a sign to a given protein-protein interaction. Vinayagam et al. who devised this technique state that a minimum of nine RNAi screens are required with confidence increasing as one carries out more screens.[59]
As therapeutic targets
Modulation of PPI is challenging and is receiving increasing attention by the scientific community.[61] Several properties of PPI such as allosteric sites and hotspots, have been incorporated into drug-design strategies.[62][63] The relevance of PPI as putative therapeutic targets for the development of new treatments is particularly evident in cancer, with several ongoing clinical trials within this area.
The consensus among these promising targets is, nonetheless, denoted in the already available drugs on the market to treat a multitude of diseases. Examples are Titrobifan, inhibitor of the glycoprotein IIb/IIIa, used as a cardiovascular drug, and Maraviroc, inhibitor of the CCR5-gp120 interaction, used as anti-HIV drug.[64]
Recently,[when?] Amit Jaiswal and others were able to develop 30 peptides using protein–protein interaction studies to inhibit telomerase recruitment towards telomeres.[65][66]
See also
- 3did
- Allostery
- Biological network
- Biological machines
- Enzyme catalysis
- Human interactome
- Multiprotein complex
- Protein domain dynamics
- Protein flexibility
- Protein structure
- Protein–protein interaction prediction
- Protein–protein interaction screening
- Systems biology
References
^ abcdefgh De Las Rivas J, Fontanillo C (June 2010). "Protein-protein interactions essentials: key concepts to building and analyzing interactome networks". PLoS Computational Biology. 6 (6): e1000807. Bibcode:2010PLSCB...6E0807D. doi:10.1371/journal.pcbi.1000807. PMC 2891586 . PMID 20589078.
. PMID 20589078.
^ Herce HD, Deng W, Helma J, Leonhardt H, Cardoso MC (2013). "Visualization and targeted disruption of protein interactions in living cells". Nature Communications. 4: 2660. Bibcode:2013NatCo...4E2660H. doi:10.1038/ncomms3660. PMC 3826628 . PMID 24154492.
. PMID 24154492.
^ Hanukoglu I (1996). "Electron transfer proteins of cytochrome P450 systems" (PDF). Adv. Mol. Cell Biol. 14: 29–55. doi:10.1016/S1569-2558(08)60339-2.
^ Brandt ME, Vickery LE (1993). "Charge pair interactions stabilizing ferredoxin-ferredoxin reductase complexes. Identification by complementary site-specific mutations". J Biol Chem. 268 (23): 17126–30. PMID 8349601.
^ Hanukoglu I (2017). "Conservation of the Enzyme-Coenzyme Interfaces in FAD and NADP Binding Adrenodoxin Reductase-A Ubiquitous Enzyme". Journal of Molecular Evolution. 85 (5): 205–218. Bibcode:2017JMolE..85..205H. doi:10.1007/s00239-017-9821-9. PMID 29177972.
^ Cooper G (2000). The cell : a molecular approach (2nd ed.). Washington DC: ASM Press. ISBN 0-87893-106-6. [page needed]
^ MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ (April 2013). "Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 304 (8): R644–50. doi:10.1152/ajpregu.00418.2012. PMC 3627954 . PMID 23408028.
. PMID 23408028.
^ abcde Jones S, Thornton JM (January 1996). "Principles of protein-protein interactions". Proceedings of the National Academy of Sciences of the United States of America. 93 (1): 13–20. Bibcode:1996PNAS...93...13J. doi:10.1073/pnas.93.1.13. PMC 40170 . PMID 8552589.
. PMID 8552589.
^ Qin K, Sethi PR, Lambert NA (August 2008). "Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins". FASEB Journal. 22 (8): 2920–7. doi:10.1096/fj.08-105775. PMC 2493464 . PMID 18434433.
. PMID 18434433.
^ Qin K, Dong C, Wu G, Lambert NA (August 2011). "Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers". Nature Chemical Biology. 7 (10): 740–7. doi:10.1038/nchembio.642. PMC 3177959 . PMID 21873996.
. PMID 21873996.
^ Malhis N & Gsponer J (June 2015). "Computational identification of MoRFs in protein sequences". Bioinformatics. 31 (11): 1738–44. doi:10.1093/bioinformatics/btv060. PMC 4443681 . PMID 25637562.
. PMID 25637562.
^ ab Westermarck J, Ivaska J, Corthals GL (July 2013). "Identification of protein interactions involved in cellular signaling". Molecular & Cellular Proteomics. 12 (7): 1752–63. doi:10.1074/mcp.R113.027771. PMC 3708163 . PMID 23481661.
. PMID 23481661.
^ Janin J (December 1999). "Wet and dry interfaces: the role of solvent in protein-protein and protein-DNA recognition". Structure. 7 (12): R277–9. doi:10.1016/s0969-2126(00)88333-1. PMID 10647173.
^ Barillari C, Taylor J, Viner R, Essex JW (March 2007). "Classification of water molecules in protein binding sites". Journal of the American Chemical Society. 129 (9): 2577–87. doi:10.1021/ja066980q. PMID 17288418.
^ Lisova O, Belkadi L, Bedouelle H (April 2014). "Direct and indirect interactions in the recognition between a cross-neutralizing antibody and the four serotypes of dengue virus". Journal of Molecular Recognition. 27 (4): 205–14. doi:10.1002/jmr.2352. PMID 24591178.
^ England P, Brégégère F, Bedouelle H (January 1997). "Energetic and kinetic contributions of contact residues of antibody D1.3 in the interaction with lysozyme". Biochemistry. 36 (1): 164–72. doi:10.1021/bi961419y. PMID 8993330.
^ ab Janin J, Chothia C (September 1990). "The structure of protein-protein recognition sites". The Journal of Biological Chemistry. 265 (27): 16027–30. PMID 2204619.
^ ab Bruce A, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1. [page needed]
^ Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC (March 1958). "A three-dimensional model of the myoglobin molecule obtained by x-ray analysis". Nature. 181 (4610): 662–6. Bibcode:1958Natur.181..662K. doi:10.1038/181662a0. PMID 13517261.
^ Cooper DR, Porebski PJ, Chruszcz M, Minor W (August 2011). "X-ray crystallography: Assessment and validation of protein-small molecule complexes for drug discovery". Expert Opinion on Drug Discovery. 6 (8): 771–782. doi:10.1517/17460441.2011.585154. PMC 3138648 . PMID 21779303.
. PMID 21779303.
^ Wand AJ, Englander SW (August 1996). "Protein complexes studied by NMR spectroscopy". Current Opinion in Biotechnology. 7 (4): 403–8. doi:10.1016/s0958-1669(96)80115-7. PMC 3442359 . PMID 8768898.
. PMID 8768898.
^ Vinogradova O, Qin J (2012). "NMR as a unique tool in assessment and complex determination of weak protein-protein interactions". Topics in Current Chemistry. 326: 35–45. doi:10.1007/128_2011_216. PMC 3676910 . PMID 21809187.
. PMID 21809187.
^ abcdefgh Berridge, M.J. (2012). "Cell Signalling Biology: Module 6 – Spatial and Temporal Aspects of Signalling". Biochemical Journal. doi:10.1042/csb0001006.
^ Yan C, Wu F, Jernigan RL, Dobbs D, Honavar V (January 2008). "Characterization of protein-protein interfaces". The Protein Journal. 27 (1): 59–70. doi:10.1007/s10930-007-9108-x. PMC 2566606 . PMID 17851740.
. PMID 17851740.
^ Jones S, Thornton JM (September 1997). "Analysis of protein-protein interaction sites using surface patches". Journal of Molecular Biology. 272 (1): 121–32. doi:10.1006/jmbi.1997.1234. PMID 9299342.
^ Chothia C, Janin J (August 1975). "Principles of protein-protein recognition". Nature. 256 (5520): 705–8. Bibcode:1975Natur.256..705C. doi:10.1038/256705a0. PMID 1153006.
^ Qin K, Dong C, Wu G, Lambert NA (August 2011). "Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers". Nature Chemical Biology. 7 (10): 740–7. doi:10.1038/nchembio.642. PMC 3177959 . PMID 21873996.
. PMID 21873996.
^ Phizicky EM, Fields S (March 1995). "Protein-protein interactions: methods for detection and analysis". Microbiological Reviews. 59 (1): 94–123. PMC 239356 . PMID 7708014.
. PMID 7708014.
^ Terentiev AA, Moldogazieva NT, Shaitan KV (December 2009). "Dynamic proteomics in modeling of the living cell. Protein-protein interactions". Biochemistry. Biokhimiia. 74 (13): 1586–607. doi:10.1134/s0006297909130112. PMID 20210711.
^ abc Wodak SJ, Vlasblom J, Turinsky AL, Pu S (December 2013). "Protein-protein interaction networks: the puzzling riches". Current Opinion in Structural Biology. 23 (6): 941–53. doi:10.1016/j.sbi.2013.08.002. PMID 24007795.
^ Rajagopala SV, Sikorski P, Caufield JH, Tovchigrechko A, Uetz P (December 2012). "Studying protein complexes by the yeast two-hybrid system". Methods. 58 (4): 392–9. doi:10.1016/j.ymeth.2012.07.015. PMC 3517932 . PMID 22841565.
. PMID 22841565.
^ ab Stelzl U, Wanker EE (December 2006). "The value of high quality protein-protein interaction networks for systems biology". Current Opinion in Chemical Biology. 10 (6): 551–8. doi:10.1016/j.cbpa.2006.10.005. PMID 17055769.
^ abc Petschnigg J, Snider J, Stagljar I (February 2011). "Interactive proteomics research technologies: recent applications and advances". Current Opinion in Biotechnology. 22 (1): 50–8. doi:10.1016/j.copbio.2010.09.001. PMID 20884196.
^ Venkatesan K, Rual JF, Vazquez A, Stelzl U, Lemmens I, Hirozane-Kishikawa T, Hao T, Zenkner M, Xin X, Goh KI, Yildirim MA, Simonis N, Heinzmann K, Gebreab F, Sahalie JM, Cevik S, Simon C, de Smet AS, Dann E, Smolyar A, Vinayagam A, Yu H, Szeto D, Borick H, Dricot A, Klitgord N, Murray RR, Lin C, Lalowski M, Timm J, Rau K, Boone C, Braun P, Cusick ME, Roth FP, Hill DE, Tavernier J, Wanker EE, Barabási AL, Vidal M (2009). "An empirical framework for binary interactome mapping". Nat Methods. 6: 83–90. doi:10.1038/nmeth.1280. PMC 2872561 . PMID 19060904.
. PMID 19060904.
^ Battesti A, Bouveret E (December 2012). "The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli". Methods. 58 (4): 325–34. doi:10.1016/j.ymeth.2012.07.018. PMID 22841567.
^ Brettner LM, Masel J (September 2012). "Protein stickiness, rather than number of functional protein-protein interactions, predicts expression noise and plasticity in yeast". BMC Systems Biology. 6: 128. doi:10.1186/1752-0509-6-128. PMC 3527306 . PMID 23017156.
. PMID 23017156.
^ Badal VD, Kundrotas PJ, Vakser IA (December 2015). "Text Mining for Protein Docking". PLoS Computational Biology. 11 (12): e1004630. Bibcode:2015PLSCB..11E4630B. doi:10.1371/journal.pcbi.1004630. PMC 4674139 . PMID 26650466.
. PMID 26650466.
^ Papanikolaou N, Pavlopoulos GA, Theodosiou T, Iliopoulos I (March 2015). "Protein-protein interaction predictions using text mining methods". Methods. Text mining of biomedical literature. 74: 47–53. doi:10.1016/j.ymeth.2014.10.026. PMID 25448298.
^ abc Ganapathiraju MK, Thahir M, Handen A, Sarkar SN, Sweet RA, Nimgaonkar VL, Loscher CE, Bauer EM, Chaparala S (April 2016). "Schizophrenia interactome with 504 novel protein-protein interactions". NPJ Schizophrenia. 2: 16012. doi:10.1038/npjschz.2016.12. PMC 4898894 . PMID 27336055.
. PMID 27336055.
^ ab Qi Y, Dhiman HK, Bhola N, Budyak I, Kar S, Man D, Dutta A, Tirupula K, Carr BI, Grandis J, Bar-Joseph Z, Klein-Seetharaman J (December 2009). "Systematic prediction of human membrane receptor interactions". Proteomics. 9 (23): 5243–55. doi:10.1002/pmic.200900259. PMC 3076061 . PMID 19798668.
. PMID 19798668.
^ Qi Y, Bar-Joseph Z, Klein-Seetharaman J (May 2006). "Evaluation of different biological data and computational classification methods for use in protein interaction prediction". Proteins. 63 (3): 490–500. doi:10.1002/prot.20865. PMC 3250929 . PMID 16450363.
. PMID 16450363.
^ Xenarios I, Rice DW, Salwinski L, Baron MK, Marcotte EM, Eisenberg D (January 2000). "DIP: the database of interacting proteins". Nucleic Acids Research. 28 (1): 289–91. doi:10.1093/nar/28.1.289. PMC 102387 . PMID 10592249.
. PMID 10592249.
^ Alonso-López D, Gutiérrez MA, Lopes KP, Prieto C, Santamaría R, De Las Rivas J (July 2016). "APID interactomes: providing proteome-based interactomes with controlled quality for multiple species and derived networks". Nucleic Acids Research. 44 (W1): W529–35. doi:10.1093/nar/gkw363. PMC 4987915 . PMID 27131791.
. PMID 27131791.
^ Goll J, Rajagopala SV, Shiau SC, Wu H, Lamb BT, Uetz P (August 2008). "MPIDB: the microbial protein interaction database". Bioinformatics. 24 (15): 1743–4. doi:10.1093/bioinformatics/btn285. PMC 2638870 . PMID 18556668.
. PMID 18556668.
^ Orii N, Ganapathiraju MK (2012). "Wiki-pi: a web-server of annotated human protein-protein interactions to aid in discovery of protein function". PLOS One. 7 (11): e49029. doi:10.1371/journal.pone.0049029. PMID 23209562.
^ McDowall MD, Scott MS, Barton GJ (January 2009). "PIPs: human protein-protein interaction prediction database". Nucleic Acids Research. 37 (Database issue): D651–6. doi:10.1093/nar/gkn870. PMID 18988626.
^ Schwikowski B, Uetz P, Fields S (December 2000). "A network of protein-protein interactions in yeast". Nature Biotechnology. 18 (12): 1257–61. doi:10.1038/82360. PMID 11101803.
^ Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Séraphin B (October 1999). "A generic protein purification method for protein complex characterization and proteome exploration". Nature Biotechnology. 17 (10): 1030–2. doi:10.1038/13732. PMID 10504710.
^ Prieto C, De Las Rivas J (July 2006). "APID: Agile Protein Interaction DataAnalyzer". Nucleic Acids Research. 34 (Web Server issue): W298–302. doi:10.1093/nar/gkl128. PMC 1538863 . PMID 16845013.
. PMID 16845013.
^ Kohl M, Wiese S, Warscheid B (2011). "Cytoscape: Software for Visualization and Analysis of Biological Networks". Data Mining in Proteomics. Methods in Molecular Biology. 696. pp. 291–303. doi:10.1007/978-1-60761-987-1_18. ISBN 978-1-60761-986-4.
^ Raman K (February 2010). "Construction and analysis of protein-protein interaction networks". Automated Experimentation. 2 (1): 2. doi:10.1186/1759-4499-2-2. PMC 2834675 . PMID 20334628.
. PMID 20334628.
^ Ideker T, Ozier O, Schwikowski B, Siegel AF (2002-01-01). "Discovering regulatory and signalling circuits in molecular interaction networks". Bioinformatics. 18 Suppl 1: S233–40. doi:10.1093/bioinformatics/18.suppl_1.s233. PMID 12169552.
^ Ayati M, Erten S, Chance MR, Koyutürk M (2015-06-30). "MOBAS: identification of disease-associated protein subnetworks using modularity-based scoring". EURASIP Journal on Bioinformatics and Systems Biology. 2015 (1): 1–14. doi:10.1186/s13637-015-0025-6. ISSN 1687-4153.
^ Rivas M, Villar D, González P, Dopazo XM, Mellstrom B, Naranjo JR (August 2011). "Building the DREAM interactome". Science China. Life Sciences. 54 (8): 786–92. doi:10.1007/s11427-011-4196-4. PMID 21786202.
^ Esteves SL, Domingues SC, da Cruz e Silva OA, Fardilha M, da Cruz e Silva EF (2012). "Protein phosphatase 1α interacting proteins in the human brain". OMICS. 16 (1–2): 3–17. doi:10.1089/omi.2011.0041. PMC 3275796 . PMID 22321011.
. PMID 22321011.
^ De Domenico M, Nicosia V, Arenas A, Latora V (April 2015). "Structural reducibility of multilayer networks". Nature Communications. 6: 6864. Bibcode:2015NatCo...6E6864D. doi:10.1038/ncomms7864. PMID 25904309.
^ Fischer B, Sandmann T, Horn T, Billmann M, Chaudhary V, Huber W, Boutros M (March 2015). "A map of directional genetic interactions in a metazoan cell". eLife. 4. doi:10.7554/eLife.05464. PMC 4384530 . PMID 25748138.
. PMID 25748138.
^ Ideker T., Tan K. & Uetz P. (2005) Visualization and integration of protein-protein interactions. In: Golemis, E. (ed.) Protein-Protein Interactions – A Molecular Cloning Manual, 2nd ed. Cold Spring Harbor Laboratory Press.
^ ab Vinayagam A, Zirin J, Roesel C, Hu Y, Yilmazel B, Samsonova AA, Neumüller RA, Mohr SE, Perrimon N (January 2014). "Integrating protein-protein interaction networks with phenotypes reveals signs of interactions". Nature Methods. 11 (1): 94–9. doi:10.1038/nmeth.2733. PMC 3877743 . PMID 24240319.
. PMID 24240319.
^ Chen GI, Gingras AC (July 2007). "Affinity-purification mass spectrometry (AP-MS) of serine/threonine phosphatases". Methods. 42 (3): 298–305. doi:10.1016/j.ymeth.2007.02.018. PMID 17532517.
^ Laraia L, McKenzie G, Spring DR, Venkitaraman AR, Huggins DJ (June 2015). "Overcoming Chemical, Biological, and Computational Challenges in the Development of Inhibitors Targeting Protein-Protein Interactions". Chemistry & Biology. 22 (6): 689–703. doi:10.1016/j.chembiol.2015.04.019. PMC 4518475 . PMID 26091166.
. PMID 26091166.
^ Arkin MR, Wells JA (April 2004). "Small-molecule inhibitors of protein-protein interactions: progressing towards the dream". Nature Reviews. Drug Discovery. 3 (4): 301–17. doi:10.1038/nrd1343. PMID 15060526.
^ Chen J, Sawyer N, Regan L (April 2013). "Protein-protein interactions: general trends in the relationship between binding affinity and interfacial buried surface area". Protein Science. 22 (4): 510–5. doi:10.1002/pro.2230. PMC 3610057 . PMID 23389845.
. PMID 23389845.
^ Ivanov AA, Khuri FR, Fu H (July 2013). "Targeting protein-protein interactions as an anticancer strategy". Trends in Pharmacological Sciences. 34 (7): 393–400. doi:10.1016/j.tips.2013.04.007. PMC 3773978 . PMID 23725674.
. PMID 23725674.
^ Jaiswal A, Lakshmi PT (9 September 2014). "Molecular inhibition of telomerase recruitment using designer peptides: an in silico approach". Journal of Biomolecular Structure & Dynamics. 33 (7): 1442–59. doi:10.1080/07391102.2014.953207. PMID 25204447.
^ Jaiswal, Amit (2014). "AtTRB1–3 Mediates Structural Changes in AtPOT1b to Hold ssDNA". ISRN Structural Biology. 2014: 1–16. doi:10.1155/2014/827201.
Further reading
.mw-parser-output .refbeginfont-size:90%;margin-bottom:0.5em.mw-parser-output .refbegin-hanging-indents>ullist-style-type:none;margin-left:0.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>ddmargin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none.mw-parser-output .refbegin-100font-size:100%
Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, Tyers M (January 2006). "BioGRID: a general repository for interaction datasets". Nucleic Acids Research. 34 (Database issue): D535–9. doi:10.1093/nar/gkj109. PMC 1347471 . PMID 16381927.
. PMID 16381927.
Peri S, Navarro JD, Kristiansen TZ, Amanchy R, Surendranath V, Muthusamy B, Gandhi TK, Chandrika KN, Deshpande N, Suresh S, Rashmi BP, Shanker K, Padma N, Niranjan V, Harsha HC, Talreja N, Vrushabendra BM, Ramya MA, Yatish AJ, Joy M, Shivashankar HN, Kavitha MP, Menezes M, Choudhury DR, Ghosh N, Saravana R, Chandran S, Mohan S, Jonnalagadda CK, Prasad CK, Kumar-Sinha C, Deshpande KS, Pandey A (January 2004). "Human protein reference database as a discovery resource for proteomics". Nucleic Acids Research. 32 (Database issue): D497–501. doi:10.1093/nar/gkh070. PMC 308804 . PMID 14681466.
. PMID 14681466.
Hermjakob H, Montecchi-Palazzi L, Lewington C, Mudali S, Kerrien S, Orchard S, Vingron M, Roechert B, Roepstorff P, Valencia A, Margalit H, Armstrong J, Bairoch A, Cesareni G, Sherman D, Apweiler R (January 2004). "IntAct: an open source molecular interaction database". Nucleic Acids Research. 32 (Database issue): D452–5. doi:10.1093/nar/gkh052. PMC 308786 . PMID 14681455.
. PMID 14681455.
Chatr-aryamontri A, Ceol A, Palazzi LM, Nardelli G, Schneider MV, Castagnoli L, Cesareni G (January 2007). "MINT: the Molecular INTeraction database". Nucleic Acids Research. 35 (Database issue): D572–4. doi:10.1093/nar/gkl950. PMC 1751541 . PMID 17135203.
. PMID 17135203.
Güldener U, Münsterkötter M, Oesterheld M, Pagel P, Ruepp A, Mewes HW, Stümpflen V (January 2006). "MPact: the MIPS protein interaction resource on yeast". Nucleic Acids Research. 34 (Database issue): D436–41. doi:10.1093/nar/gkj003. PMC 1347366 . PMID 16381906.
. PMID 16381906.
Pagel P, Kovac S, Oesterheld M, Brauner B, Dunger-Kaltenbach I, Frishman G, Montrone C, Mark P, Stümpflen V, Mewes HW, Ruepp A, Frishman D (March 2005). "The MIPS mammalian protein-protein interaction database". Bioinformatics. 21 (6): 832–4. doi:10.1093/bioinformatics/bti115. PMID 15531608.
External links
| Wikimedia Commons has media related to Protein interaction mapping. |
- Protein-Protein Interaction Databases
- Library of Modulators of Protein–Protein Interactions (PPI)
Proteins and Enzymes at Curlie (based on DMOZ)
Casado-Vela J, Matthiesen R, Sellés S, Naranjo JR (May 2013). "Protein-Protein Interactions: Gene Acronym Redundancies and Current Limitations Precluding Automated Data Integration". Proteomes. 1 (1): 3–24. doi:10.3390/proteomes1010003. PMID 28250396.

 Clash Royale CLAN TAG#URR8PPP
Clash Royale CLAN TAG#URR8PPP